Every chemistry student has to learn how to draw Lewis Dot Structures. The key is to understand the steps and practice. Lewis Structures are important to learn because they help us predict: the shape of a molecule. How the molecule might react with other molecules. The physical properties of the molecule (like boiling point, surface tension, etc.). This demo will convert a skeletal figure, provided by a drawing in the HTML5 SketcherCanvas component on the left, into a Lewis Dot Structure in the Canvas on the right. When you are finished drawing your 2D structure, click on the Get Lewis Dot Structure button to see the result. This feature is customizable with publication quality graphics output in ChemDoodle 2D. In this lecture, I'll be discussing Lewis Dot Symbols for Elements which show the valence electrons. From there, the lecture will move to the process of forming molecules, and depicting chemical bonds using Lewis Dot structures. Along the way I'll introduce bond dipoles. Here is a picture of a couple of hydrogen atoms. It's a very simple drawing. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures.
- Lewis Dot Structure Chemistry
- Lewis Structure Calculator
- Chemistry Lewis Dot Structure Calculator Online
South central cartel discography torrent. A bond is the sharing of 2 electrons. Covalent bonds share electrons in order to form a stable octet around each atom in the molecules. Hydrogen is the exception it only requires 2 electrons (a duet) to be stable. How do we draw a covalent Lewis Dot Structure? Level 1 (basic) 1. Add up all the valance electrons of the atoms involved. ex CF4 So C has 4 and F has 7 (x4 we have 4Fs) = 32 valence electrons 2. You need to pick the central atom. This is usually easy, this atom will be surrounded by the others. Never H. So C will be surrounded by F's. https://suffputcuba.tistory.com/8. 3. Now we create our skeleton structure by placing bonds in. A bond is a dash that represents 2 electrons. We have now placed 8 electrons as 4 bonds. We have 32-8= 24 more to place. 4. Starting with the outer atoms add the remaining electrons in pairs until all the electrons have run out.
All 32 electrons are now in place, count the dots around each F. 6 dots and a bond (2 electrons) is 8. We have our octet. The carbon has 4 bonds (2electrons) for its 8. DONE Level 2 (Double and Triple bonds) Same rules apply until #4 1. Add up all the valance electrons of the atoms involved. ex CO2 So C has 4 and O has 6 (x2 ) = 16 valence electrons 2. You need to pick the central atom. This is usually easy, this atom will be surrounded by the others. Never H. So C will be surrounded by O's. 3. Now we create our skeleton structure by placing bonds in. A bond is a dash that represents 2 electrons. We have now placed 4 electrons as 2 bonds. We have 16-4=12 more to place. 4. Starting with the outer atoms add the remaining electrons in pairs until all the electrons have run out.
All 16 electrons are now in place, count the dots around each O. 6 dots and a bond (2 electrons) is 8. We have our octet. The carbon has 2 bonds (2electrons) for its 4..? We need 8, so move a pair of electrons from the O to between the C and O. It will share 2 pairs of electrons instead of 1. It now has a double bond instead of a single bond.
now they all have an octet, it cleans up like this Make it symmetrical. Level 3-Lewis Dots of Polyatomic Ions Same rules apply, at the end they get brackets and a charge AP Chemistry and or College Level Rules 1. Determine whether the compound is covalent or ionic. If covalent, treat the entire molecule. If ionic, treat each ion separately. Compounds of low electronegativity metals with high electronegativity nonmetals (DEN > 1.7) are ionic as are compounds of metals with polyatomic anions. For a monoatomic ion, the electronic configuration of the ion represents the correct Lewis structure. For compounds containing complex ions, you must learn to recognize the formulas of cations and anions. 2. Determine the total number of valence electrons available to the molecule or ion by:
3. Organize the atoms so there is a central atom (usually the least electronegative) surrounded by ligand (outer) atoms. Hydrogen is never the central atom. https://suffputcuba.tistory.com/7. 4. Determine a provisional electron distribution by arranging the electron pairs (E.P.) in the following manner until all available pairs have been distributed:
5. Calculate the formal charge (F) on the central atom.
6. If the central atom formal charge is zero or is equal to the charge on the species, the provisional electron distribution from (4) is correct. Calculate the formal charge of the ligand atoms to complete the Lewis structure. 7. If the structure is not correct, calculate the formal charge on each of the ligand atoms. Then to obtain the correct structure, form a multiple bond by sharing an electron pair from the ligand atom that has the most negative formal charge.
8. Recalculate the formal charge of each atom to complete the Lewis structure. on to Formal Charge Chemical Demonstration Videos |
Learning Objectives
- State the octet rule.
- Define ionic bond.
- Draw Lewis structures for ionic compounds.
In Section 4.7 we saw how ions are formed by losing electrons to make cations or by gaining electrons to form anions. The astute reader may have noticed something: many of the ions that form have eight electrons in their valence shell. Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons in their original valence shell; the lower shell, now the valence shell, has eight electrons in it, so the atom becomes positively charged. For whatever reason, having eight electrons in a valence shell is a particularly energetically stable arrangement of electrons. The octet rule explains the favorable trend of atoms having eight electrons in their valence shell. When atoms form compounds, the octet rule is not always satisfied for all atoms at all times, but it is a very good rule of thumb for understanding the kinds of bonding arrangements that atoms can make.
It is not impossible to violate the octet rule. Consider sodium: in its elemental form, it has one valence electron and is stable. It is rather reactive, however, and does not require a lot of energy to remove that electron to make the Na+ ion. We could remove another electron by adding even more energy to the ion, to make the Na2+ ion. However, that requires much more energy than is normally available in chemical reactions, so sodium stops at a 1+ charge after losing a single electron. It turns out that the Na+ ion has a complete octet in its new valence shell, the n = 2 shell, which satisfies the octet rule. The octet rule is a result of trends in energies and is useful in explaining why atoms form the ions that they do.
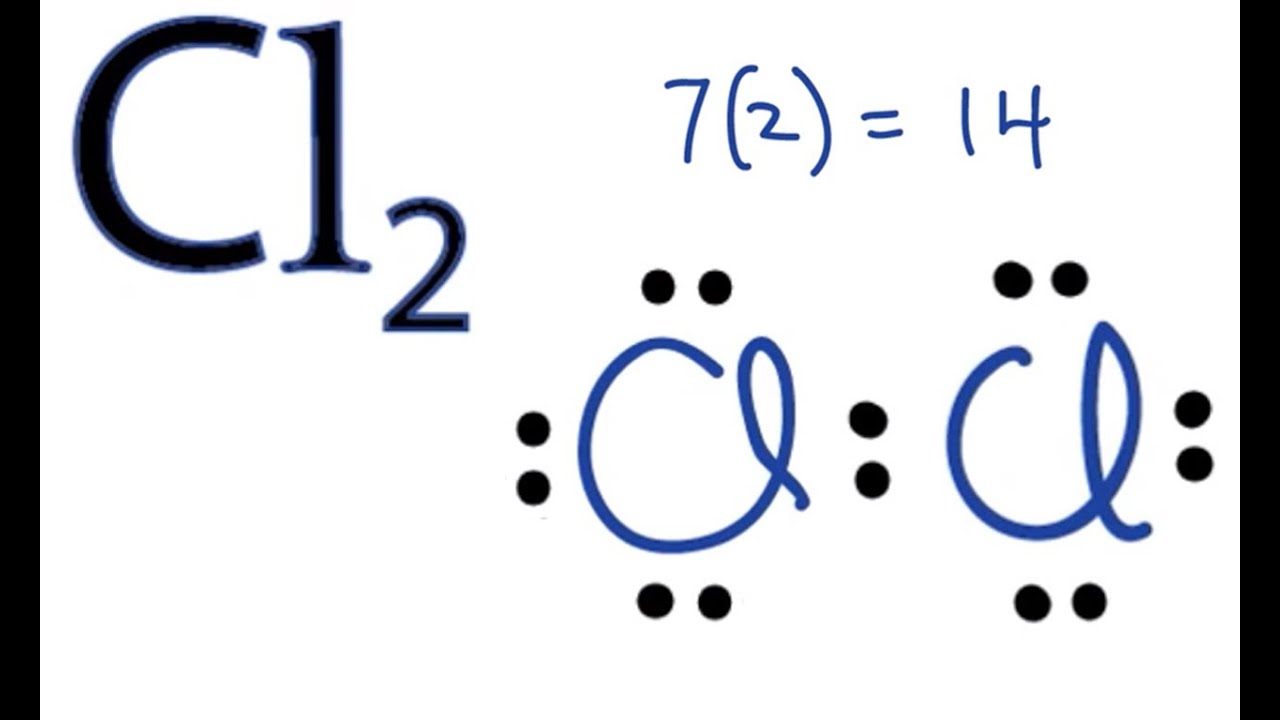
Now consider an Na atom in the presence of a Cl atom. The two atoms have these Lewis electron dot diagrams and electron configurations:
[mathbf{Na, cdot }; ; ; ; ; ; ; ; ; ; mathbf{cdot }mathbf{ddot{underset{.: .}Cl}}mathbf{: :}]
Lewis Dot Structure Chemistry

[left [ Ne right ]3s^{1}; ; ; ; left [ Ne right ]3s^{2}3p^{5}]
For the Na atom to obtain an octet, it must lose an electron; for the Cl atom to gain an octet, it must gain an electron. An electron transfers from the Na atom to the Cl atom:

[mathbf{Na, cdot }curvearrowright mathbf{cdot }mathbf{ddot{underset{.: .}Cl}}mathbf{: :}]
resulting in two ions—the Na+ ion and the Cl− ion: Hospital inventory management software, free download.
[mathbf{Na}^{+}; ; ; ; ; ; ; ; mathbf{:}mathbf{ddot{underset{.: .}Cl}}mathbf{: :}^{-}]
[left [ Ne right ]; ; ; ; ; left [ Ne right ]3s^{2}3p^{6}]
Both species now have complete octets, and the electron shells are energetically stable. From basic physics, we know that opposite charges attract. This is what happens to the Na+ and Cl− ions:
[mathbf{Na}^{+}; + ; mathbf{:}mathbf{ddot{underset{.: .}Cl}}mathbf{: :}^{-}rightarrow Na^{+}Cl^{-}; ; or; ; NaCl]
where we have written the final formula (the formula for sodium chloride) as per the convention for ionic compounds, without listing the charges explicitly. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. Ionic bonds are caused by electrons transferring from one atom to another.
In electron transfer, the number of electrons lost must equal the number of electrons gained. We saw this in the formation of NaCl. A similar process occurs between Mg atoms and O atoms, except in this case two electrons are transferred:
The two ions each have octets as their valence shell, and the two oppositely charged particles attract, making an ionic bond:
[mathbf{Mg,}^{2+}; + ; left[mathbf{:}mathbf{ddot{underset{.: .}O}}mathbf{: :}right]^{2-}; ; ; ; ; Mg^{2+}O^{2-}; or; MgO]
Remember, in the final formula for the ionic compound, we do not write the charges on the ions.
What about when an Na atom interacts with an O atom? The O atom needs two electrons to complete its valence octet, but the Na atom supplies only one electron:
[mathbf{Na, cdot }curvearrowright mathbf{cdot }mathbf{ddot{underset{.}O}}mathbf{: :}]
The O atom still does not have an octet of electrons. What we need is a second Na atom to donate a second electron to the O atom:
These three ions attract each other to give an overall neutral-charged ionic compound, which we write as Na2O. The need for the number of electrons lost being equal to the number of electrons gained explains why ionic compounds have the ratio of cations to anions that they do. This is required by the law of conservation of matter as well.
Example (PageIndex{1}): Synthesis of calcium chloride from Elements
With arrows, illustrate the transfer of electrons to form calcium chloride from (Ca) atoms and (Cl) atoms.
Solution
Lewis Structure Calculator
A (Ca) atom has two valence electrons, while a (Cl) atom has seven electrons. A (Cl) atom needs only one more to complete its octet, while (Ca) atoms have two electrons to lose. Thus we need two (Cl) atoms to accept the two electrons from one (Ca) atom. The transfer process looks as follows:
The oppositely charged ions attract each other to make CaCl2.
Exercise (PageIndex{1})
Gparted for mac download. With arrows, illustrate the transfer of electrons to form potassium sulfide from (K) atoms and (S) atoms.
- Answer
Summary
- The tendency to form species that have eight electrons in the valence shell is called the octet rule.
- The attraction of oppositely charged ions caused by electron transfer is called an ionic bond.
- The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions.
Contributions & Attributions
Chemistry Lewis Dot Structure Calculator Online
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Marisa Alviar-Agnew (Sacramento City College)
Henry Agnew (UC Davis)